

Gene Therapies
OCU400 for Retinitis Pigmentosa
Retinitis pigmentosa (RP) encompasses a group of inherited retinal diseases characterized by peripheral vision loss and impaired night vision and affects approximately 300,000 people in the U.S. and Europe combined. The RP phenotype results from over 200 mutations in more than 100 distinct genes, and addressing this genetic diversity poses a significant scientific and financial challenge in developing effective gene therapies. The OCU400 (AAV5-hNR2E3) gene therapy platform offers a promising solution by delivering the nuclear hormone receptor NR2E3 to the retina and retinal pigment epithelium (RPE) to preserve and potentially restore retinal structure and function.
In five unique mouse models of RP, treatment with AAV5-NR2E3 by subretinal injection rescued multiple genetically diverse inherited retinal diseases (IRDs) by protecting photoreceptors from further damage after disease onset. The five RP models tested were rd1 (PDE6β-associated RP), Rho-/- and RhoP23H (both rhodopsin-associated RP), rd16 (Leber congenital amaurosis), and rd7 (Enhanced S-cone Syndrome). The studies published in Nature Gene Therapy and Scientific Reports demonstrate the potential of a novel modifier gene therapy to elicit broad-spectrum therapeutic benefits in early and intermediate stages of RP and Leber congenital amaurosis (LCA) based on animal models, showing the potential for a gene-agnostic treatment in humans.
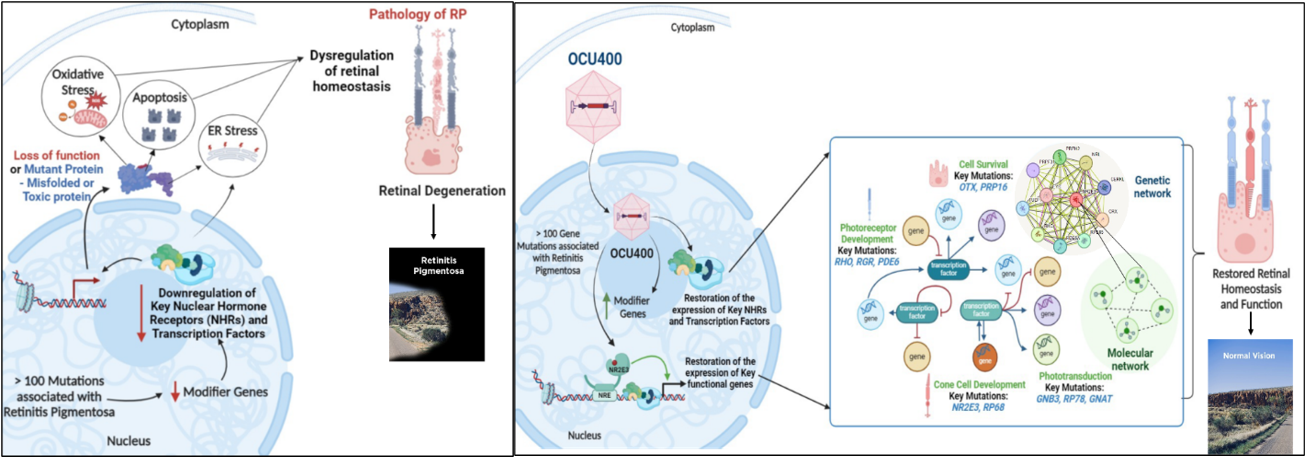
In an open-label, multi-center Phase 1/2 clinical study involving 18 subjects, treatment with OCU400 demonstrated sustained safety and tolerability. Subjects showed improvement in exploratory efficacy endpoints such as Multi-Luminance Mobility Testing (MLMT), Best-Corrected Visual Acuity (BCVA), and Low- Luminance Visual Acuity (LLVA) in the treated eye compared to baseline 12 months after subretinal dosing. Treatment-related SAEs were uncommon and predominantly related to surgical complications. Altogether, these findings suggest that subretinal delivery of OCU400 is safe and has the potential to deliver a one-time therapy for life.
Top-line results of the Phase 1/2 study include the following:
- OCU400 is generally safe and well tolerated in 18 subjects across different dose levels and mutations (autosomal dominant or biallelic autosomal recessive NR2E3 or autosomal dominant RHO).
- Efficacy measurements suggest stabilization or improvement compared to baseline in the treated eye on the Multi-Luminance Mobility Test (MLMT) regardless of mutation.
- 14 of 18 treated eyes (78%) showed stabilization or improvement.
- Post hoc analysis confirmed that 2 patients demonstrated a ceiling effect at enrollment and 2 non-responders had AEs related to the study surgery.
- Efficacy in subjects with RHO mutations supports the gene-agnostic mechanism of action of OCU400: 8 of 10 treated eyes (80%) with RHO mutations showed stabilization or improvement.
For Phase 3, the primary endpoint is performance on the Luminance Dependent Navigation Assessment (LDNA), an enhanced version of the MLMT that is more specific and sensitive and proprietary to Ocugen. The company has aligned with the FDA on clinical efficacy, and “responders” are defined as those subjects who demonstrate an improvement of ≥ 2 lux levels on the LDNA. Notably, 3 of 5 intent-to-treat (ITT) RHO subjects (60%) from the Phase 1/2 trial meet the new “responder” criteria established in collaboration with the FDA for the Phase 3 trial. 62.5% (5/8) of the ITT RHO and NR2E3 patients from the Phase 1/2 clinical trial meet the responder criteria. Assuming a response rate of only 50% (N=150, randomized 2:1), the OCU400 Phase 3 trial is powered at >95%.
Program Update:
- With dosing of the Phase 3 liMeliGhT (pronounced “limelight”) trial underway, OCU400 remains on track for the 2026 BLA and MAA approval targets.
OCU410 for Geographic Atrophy
Dry age-related macular degeneration (dAMD) affects nearly 200 million people globally and is the leading cause of irreversible sight loss among the elderly. Geographic atrophy (GA) is an advanced form of dAMD that affects approximately 2-3 million people in the U.S. and Europe combined. OCU410 (AAV5-hRORA) is a modifier gene therapy product being developed for the treatment of GA.
OCU410 utilizes an AAV platform for the subretinal delivery of the RORA (RAR-related Orphan Receptor A) gene. The RORA protein plays an important role in lipid metabolism, reducing lipofuscin deposits and oxidative stress, and demonstrates an anti-inflammatory role as well as inhibiting the complement system in both in vitro and in vivo pre-clinical studies. These results demonstrate the ability of OCU410 to target multiple pathways linked to dAMD pathophysiology. Recently approved therapies for GA focus only on the complement system, require frequent intravitreal injections (6-12 per year), and are accompanied by safety concerns with roughly 12% of patients developing the more serious wet AMD. OCU410 offers the potential of a one-time therapy for life with a single subretinal injection.
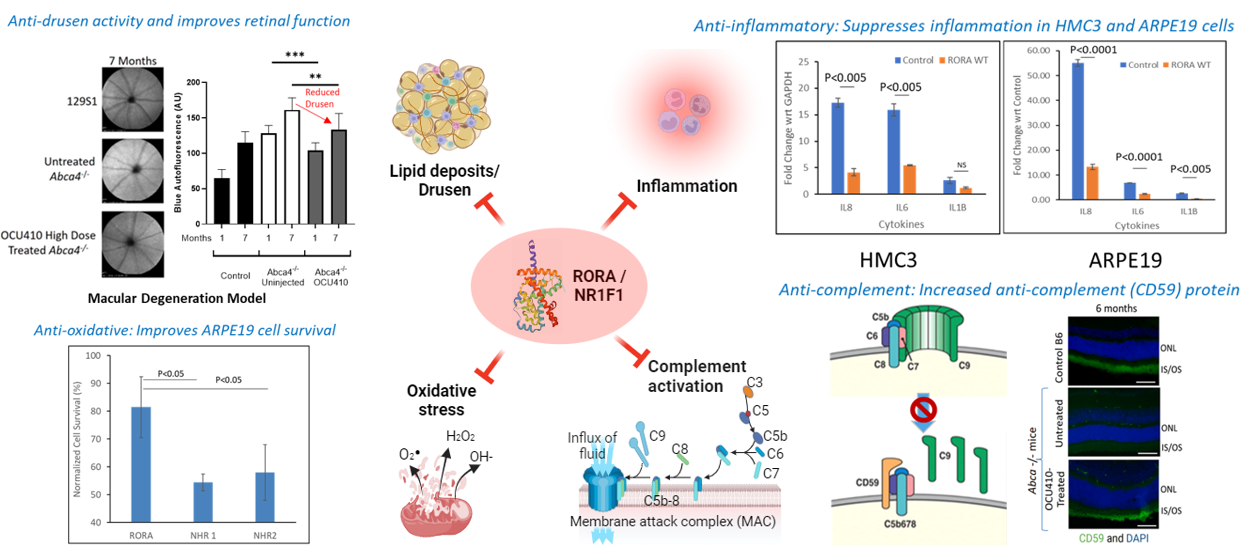
Program Update:
- Phase 2 of the ArMaDa clinical trial has been initiated and will assess the safety and efficacy of OCU410 in a larger group of patients who will be randomized into either of two treatment groups (medium- or high-dose) or a control group.
OCU410ST for Stargardt Disease
Stargardt disease affects approximately 100,000 people in the U.S. and Europe combined and is clinically characterized by slow loss of central vision from early childhood. No FDA-approved treatment is available, and current therapeutic options include photoprotection and low-vision aids. The underlying common cause involves gene mutations in the ABCA4 gene, which follows an autosomal recessive pattern of inheritance. Mutations in the ABCA4 gene lead to defective processing and transportation of all-trans retinaldehyde from the photoreceptors to retinal pigment epithelium (RPE) cells. OCU410ST (AAV5-hRORA) regulates genes and pathways involved in the onset and progression of Stargardt disease indicating its therapeutic potential.
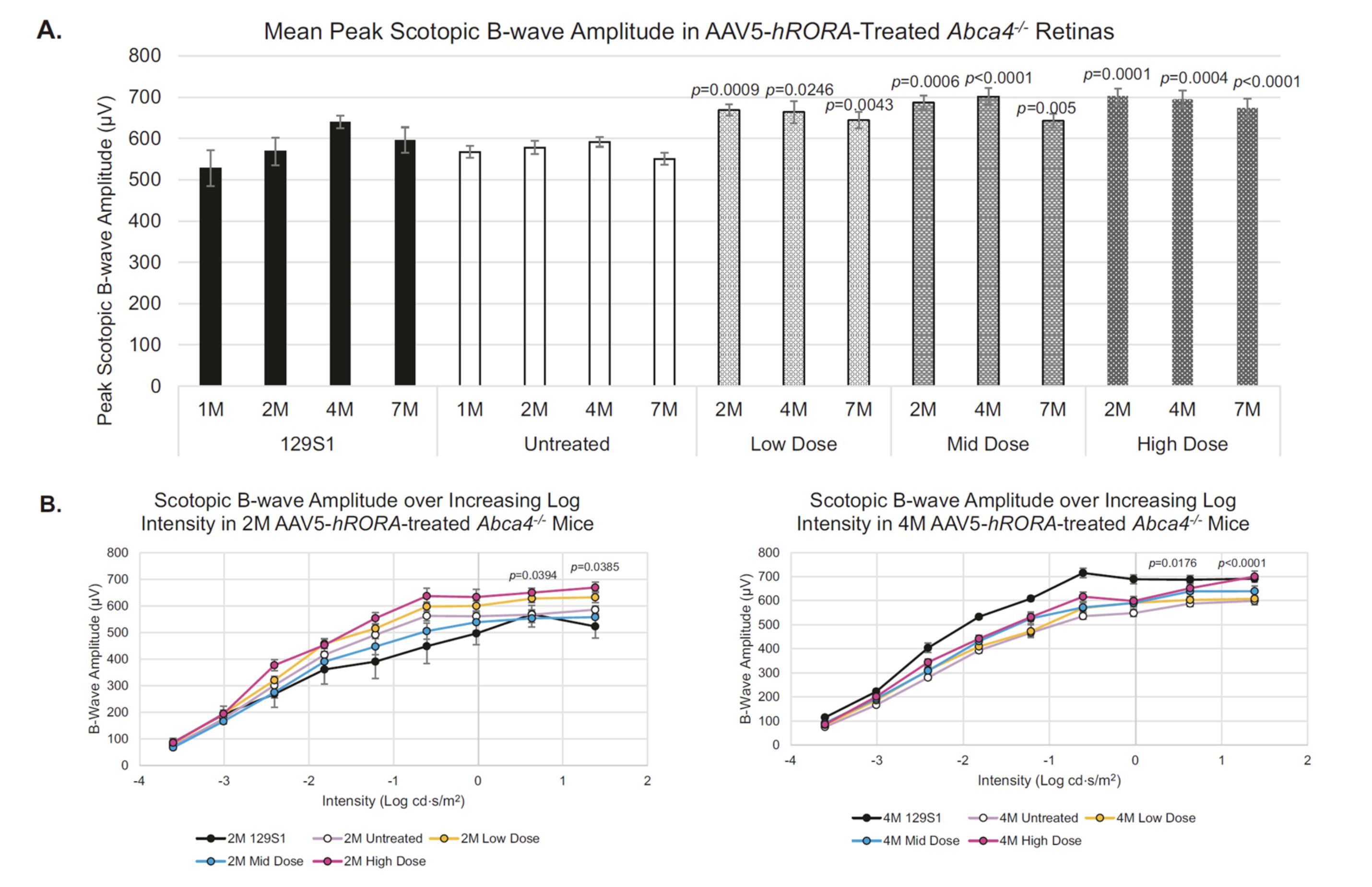
Program Update:
- Ocugen received Orphan Drug Designation from the FDA for the treatment of ABCA4-associated retinopathies including Stargardt disease, retinitis pigmentosa 19 (RP19), and cone-rod dystrophy 3 (CORD3).
- The Phase 1/2 GARDian trial is underway. The Phase 1, dose-escalation portion, is complete.

