Biologicals
OCU200
OCU200 is a biologic product candidate in preclinical development for treating severely sight-threatening diseases like Diabetic Macular Edema (DME), Diabetic Retinopathy (DR), and Wet Age-Related Macular Degeneration (Wet-AMD). Patients affected by these diseases share common symptoms, such as blurriness in vision and progressive vision loss as the disease progresses. The formation of fragile and leaky new blood vessels leads to fluid accumulation in and around the retina, causing damage to vision.
OCU200 is a novel fusion protein consisting of two human proteins, tumstatin and transferrin, that are already present normally in retinal tissues. OCU200 unique features which we believe will enable it to (a) efficiently target leaky blood vessels, (b) regress the existing abnormal blood vessels, and (c) inhibit the growth of new blood vessels in the retina and choroid. Tumstatin, which acts as an anti-VEGF, anti-inflammatory and anti-oxidative agent, is the active component of OCU200. It binds to integrin receptors, which play a crucial role in disease pathogenesis. Transferrin facilitates the targeted delivery of tumstatin into the retina and choroid and potentially helps increase the interaction between tumstatin and integrin receptors.
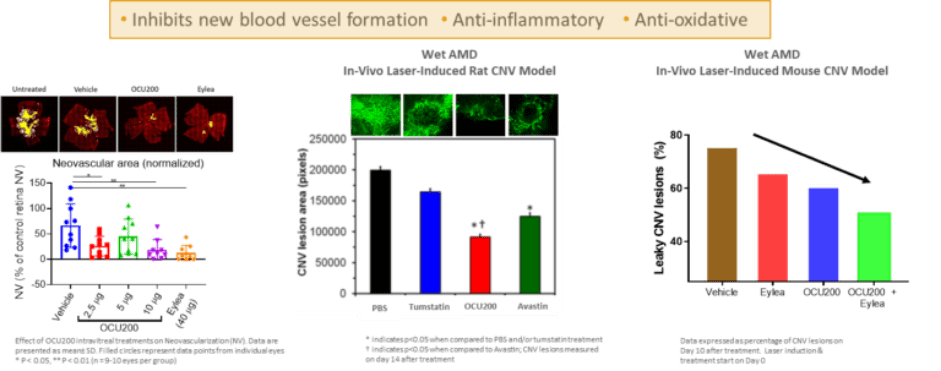
In preclinical studies, OCU200 demonstrated efficacy in different animal models of neovascularization. In an animal model for DME and DR (Oxygen-induced retinopathy in mice), OCU200 demonstrated comparable efficacy at significantly lower dose compared to existing approved anti-VEGF therapy in preventing disease manifestation and progression. In animal models for wet AMD (laser induced CNV in mice and rats), OCU200 demonstrated superior activity compared to anti-VEGF control groups in preventing the formation and growth of new leaky blood vessels and subsequent disease symptoms.
OCU200 Demonstrated Efficacy in Animal Models for DME, DR, and Wet-AMD