Vaccines
OCU500 Series
OCU500: COVID-19 inhaled vaccine
OCU510: Seasonal quadrivalent influenza inhaled vaccine
OCU520: Combination quadrivalent seasonal influenza and COVID-19 inhaled vaccine
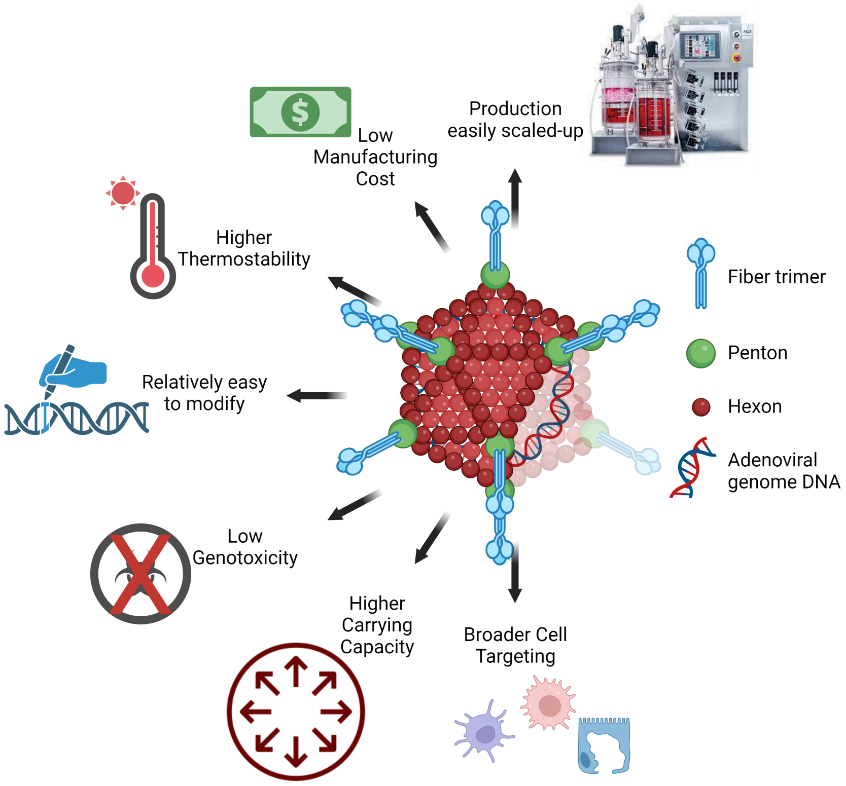
The OCU500 vaccine series is based on a novel ChAd platform designed to reduce transmission with inhalation technology as the differentiator to protect against new variants with long-term durability. The strength of the inhaled vaccine is due to the elicitation of a robust humoral (IgG) and mucosal (IgA) immune response (similar inhalation technology, CanSinoBIO’s Convedicia Air, has been approved for use in China). This method reduces dependence on needles/syringes and enables rapid administration. Multiple preclinical studies using this vector demonstrated high vaccine-induced neutralizing and effector responses. Clinical studies using a similar vector administered via the inhalation platform showed an increase in mucosal and systemic antibodies and a durable immune response up to 1 year with 1/5 of the dose compared to the same vaccine given via intramuscular administration.
Program Updates:
- FDA has endorsed the Ocugen inhaled ChAd36 COVID-19 clinical design.
- OCU500 was selected by the NIH/NIAID Project NextGen for inclusion in clinical trials. NIAID is planning to initiate Phase 1 clinical trials in 2024.

