Cell Therapies
NeoCart®
NeoCart is a three-dimensional (3D) tissue-engineered disc of new cartilage that is manufactured by growing chondrocytes – the cells responsible for maintaining cartilage health – derived from the patient on a unique scaffold. NeoCart has the potential to accelerate healing and reduce pain by rebuilding a patient’s damaged knee cartilage. It treats pain at the source, restoring the joint surface to its functional pre-injury state. Ultimately, the goal is to prevent a patient’s progression to osteoarthritis. NeoCart was acquired as a part of Ocugen’s reverse merger with the original developer of the therapy, Histogenics, in 2019.
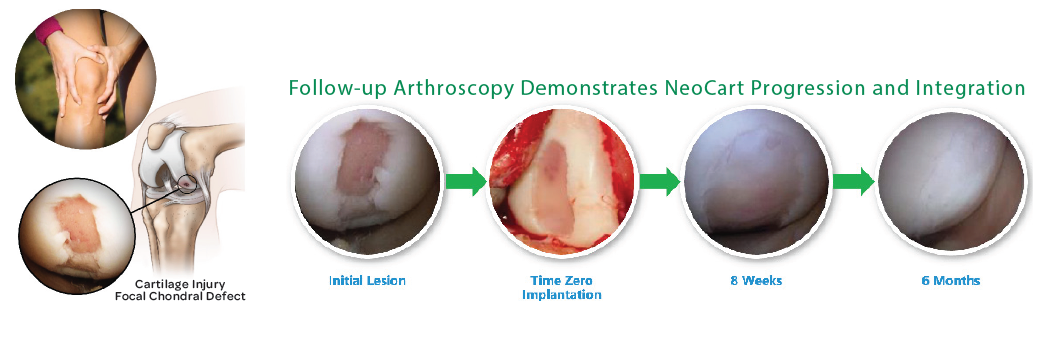
Program Updates
- NeoCart has been granted RMAT designation from FDA.
- Ocugen received FDA concurrence on a confirmatory trial design for Phase 3. Initiation is contingent upon available funding.
- Construction of the cGMP manufacturing facility is complete.

